BACKGROUND
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive subtype of lymphoma with poor prognosis. One potential therapeutic approach could involve targeting the programmed death-1 (PD-1) immune checkpoint pathway, as these tumors often express PD-L1. However, the efficacy of this strategy using sintilimab in combination with immunochemotherapy as frontline therapy for patients with newly diagnosed PCNSL remains uncertain.
METHODS
In this phase II trial with a safety run-in, we enrolled treatment-naive PCNSL patients between the ages of 18 and 70, who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. All patients were given sintilimab combined with rituximab, high-dose methotrexate and temozolomide (SRMT) regimen (six cycles every 21 days): sintilimab (200 mg, i.v., Day-0), rituximab (375 mg/m 2, i.v., Day-0), methotrexate (3.0 g/m 2, i.v., Day-1 or 1.0 g/m 2 for patients aged ≥65 years, and temozolamide (150 mg/m 2/d, p.o., Days 1-5). The primary outcome was overall response rate (ORR), with secondary outcomes including duration of response (DOR), progression-free survival (PFS), overall survival (OS), safety, and potential biomarkers.
RESULTS
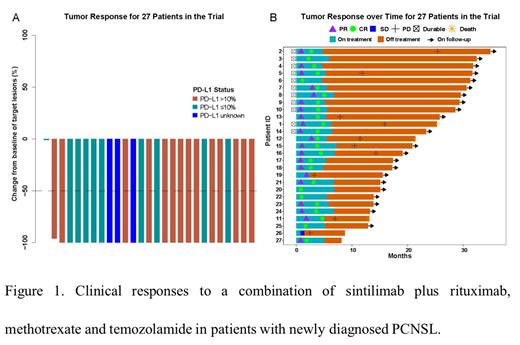
Between 3 April 2020 and 23 August 2022, a total of 28 patients were enrolled. No dose-limiting toxicities were observed during the safety run-in period. Among 27 evaluable patients, the primary outcome was met with an impressive ORR of 96.3% (95% CI: 81-99.9%). In detail, 25 patients (92.6%) achieved a complete response and one (3.7%) exhibited a partial response. As of 7 April 2023, the median follow-up was 20.8 months. The Medians for DOR, PFS, and OS were not reached. Remarkably, 2-year PFS and 2-year OS were 65.4% (48.1-88.8%) and 88.5% (74.1-100%), respectively. The most common grade 3-4 treatment-related toxicities were increased levels of alanine aminotransferase (18.5%) and aspartate aminotransferase (14.8%). Additionally, the receiver operating characteristic analysis revealed that the baseline IL10/IL6 ratio in cerebrospinal fluid exhibited promising performance in predicting PFS, achieving an area under curve of 0.91 at 2 years.
CONCLUSIONS
The SRMT regimen as frontline treatment for PCNSL is highly efficacious and well tolerated. (Chinese Clinical Trial Registry number: ChiCTR1900027433).
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Sintilimab, a humanized IgG4 monoclonal antibody, effectively inhibits PD-1 by specifically binding to the PD-1 receptor and preventing its interaction with ligands.We conducted a phase II trial with a safety run-in to evaluate the efficacy and safety of sintilimab combined with rituximab, HD-MTX, and temozolomide (SRMT) as frontline therapy for newly diagnosed PCNSL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal